how many valence electrons in chlorine|Chlorine Valence Electrons : Bacolod So how many electrons are in the third energy level? Well, there's two and five, for a total of seven. So chlorine has seven valence electrons. And once again, that's very . However, in the movie "Trailer Park Boys: Say Goodnight to The Bad Guys" Sarah finally ended the relationship because she could no longer handle Jacobs's obsession with Julian. In the movie "Trailer Park Boys: Don't Legalize It" Sarah is cheating on Lucy J-Roc, and he credits her for straightening him out and setting his priorities straight .
how many valence electrons in chlorine,This table of element valences includes the maximum valence and most . Mar 23, 2023

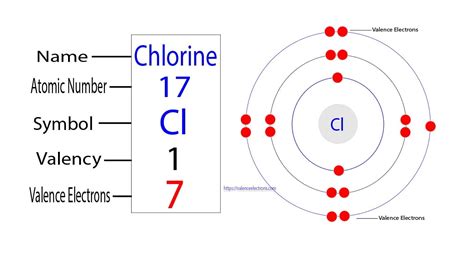
The valence electrons of chlorine are 7, the last shell of the chlorine atom has seven electrons. The valency of chlorine is 1, and the valence electrons of chloride ion (Cl –) are 8, the last shell of the . There are two ways to find the number of valence electrons in Chlorine (Cl). The first is to use the Periodic Table to figure out how many electrons Chlorine .So how many electrons are in the third energy level? Well, there's two and five, for a total of seven. So chlorine has seven valence electrons. And once again, that's very . Chlorine is in Group VIIA (Group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in Group IIA (Group 2). .how many valence electrons in chlorine Chlorine Valence Electrons Learn how to find the valence electrons and valency of chlorine, a reactive and oxidizing element with the symbol Cl and atomic number 17. The valence electrons are seven and the valency is one, as . The atomic number of chlorine is 17. Hence it has 2 electrons in its innermost shell, 8 electrons in its second shell and 7 electrons electron in the outer-most shell respectively. Titanium .
This table of element valences includes the maximum valence and most common valence values. Use this is a reference with a periodic table. . Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of .
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. . Chlorine (Cl) 7: 18: Argon (Ar) 8: 19: Potassium .
Step 4: Find Valence Electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the .
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic compounds.
The atomic number of chlorine is 17. Hence it has got 7 electrons in its outermost shell. Its valency can be found out by subtracting 7 from 8, i.e., -1. Filed Under: Period Table. Chlorine Valence Electrons or Chlorine Valency (Cl) with Dot Diagram have been available here for the students. Chlorine element symbol is cl.
There are total of seventeen electrons not valence electrons in chlorine atom. Electronic configuration: (Cl) = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5; Hence, option (C) is incorrect. (D) 18. There are 18 total electrons in the chlorine ion not valence electrons. Electronic configuration: (Cl-) = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6; Hence option (D) is incorrect.Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.

Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.how many valence electrons in chlorine Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.
Chlorine Valence Electrons Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..Determine the total number of valence electrons in chlorine molecule. The chlorine molecule contains two chlorine atoms. In the periodic table, chlorine is a group VIIA element with seven electrons in its last shell. Therefore, the total number of valence electrons= 7(2)= 14. 2. Total electron pairs exist in the form of lone pairs and bonds.Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan.All other isotopes have half-lives under 1 hour, many less than one second. Chlorine-35 is composed of 17 protons, 18 neutrons, and 17 electrons. Chlorine-37 is composed of 17 protons, 20 neutrons, and 17 electrons. .
Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1 s2 2 s2 2 p2 —that it has 4 valence electrons (2 s2 2 p2) and 2 core . Figure 5.2.1 5.2. 1 The shell structure of atoms of He, Cl, and K, as suggested by Lewis. Note the valence electrons are in red. Helium has one shell filled by two red dots. Chlorine has 2 red dots on its inner most shell, followed by 8 red dots on the next shell. The outer most shell has 7 red dots. Examples. Magnesium’s ground state electron configuration is 1s 2 2s 2 p 6 3s 2, the valence electrons would be the 3s electrons because 3 is the highest principal quantum number.Magnesium has two valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2.The highest principal quantum number is 2.Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .This means that calcium #s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. This makes calcium a Ca+2 cation. Chlorine is a Halogen in the 17th column or #s^2p^5# group. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells.
Total number of electrons of the valance shells of Cl 2 molecule. There is only one element in chlorine molecule. Chlorine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of chlorine atoms.
how many valence electrons in chlorine|Chlorine Valence Electrons
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence Electrons Chart for All Elements
PH2 · How to Find the Valence Electrons for Chlorine (Cl)?
PH3 · How to Find the Valence Electrons for Chlorine (Cl)
PH4 · How many valence electrons are in an atom of chlorine?
PH5 · How Many Valence Electrons Does Chlorine (Cl) Have? [Valency of Chlo
PH6 · How Many Valence Electrons Does Chlorine (Cl) Have?
PH7 · Determine valence electrons using the periodic table
PH8 · Counting valence electrons for main group elements
PH9 · Chlorine Valence Electrons
PH10 · 10.6: Valence Electrons